The technology of the future.
Our unique plasma source requires only electricity and ambient air to produce cold plasma products. Cold plasma products are made from electrically charged particles. They are primarily reactive oxygen compounds with oxidation potential, which eliminate harmful microorganisms. Pathogenic organisms such as bacteria, viruses and fungi are reliably oxidized. Our PLASMO® AIR and PLASMO® CAR products are based on this technology.
As part of our research, cold plasma technology was further developed into cold plasma aerosol. Cold plasma products meet previously nebulized water (aerosol). The degree of ionization of cold plasma promises a higher efficiency of the nebulized water (dispersion). The oxygen compounds of the cold plasma products also electrostatically charge the aerosol and thus increase its germreducing effect. This patented process enhances the physical dissolving and dilution effect of the aerosol. Our PLASMO® HEAL, PLASMO® VET and PLASMO® HAND products benefit from this innovative development.

Mechanism of action of cold plasma.
Cold plasma is an ionized gas. In addition to solid, liquid and gaseous, this “electrically charged” gas represents a fourth state of aggregation. Cold plasma products contained in this gas consist of various charged particles with oxidation potential. They enable the oxidation of unwanted microorganisms. The charged particles move freely in space and have their desired effect there. Cold plasma is also required to produce cold atmospheric plasma aerosol (CAP-A)). The exact explanation of cold plasma can therefore be found below in the section “Step 1. Generating Cold Atmospheric Plasma (CAP)”.
Mechanism of action of cold plasma aerosol.
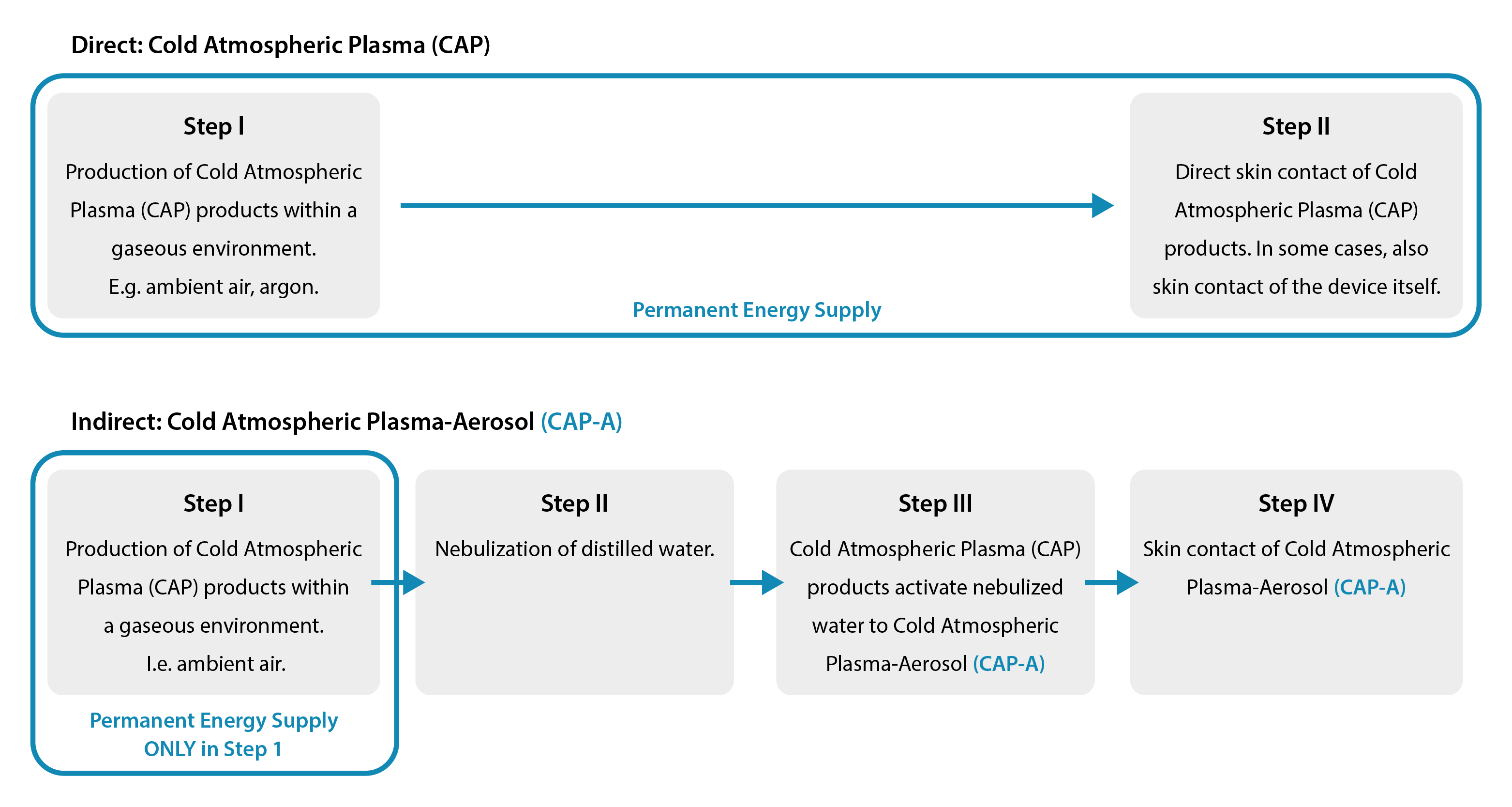
Our PLASMO® HEAL, PLASMO® VET and PLASMO® HAND products are based on the development of the direct cold plasma process. Our patented, indirect cold plasma process is referred to as “Cold Atmospheric Plasma Aerosol (CAP-A)” in scientific literature. In addition to cold plasma products, CAP-A uses the power of the aerosol. This process differs from the direct Cold Atmospheric Plasma (CAP) process because it does not require a permanent input of energy. Energy supply and destination are separated.
Step 1.
Production of Cold Atmospheric Plasma (CAP).
Plasma Disinfection Procedures for Surfaces in Emergency Service Vehicles: A Field Trial at the German Red Cross. Sci Rep 13, 20737.
DOI: https://doi.org/10.1038/s41598-023-47759-5
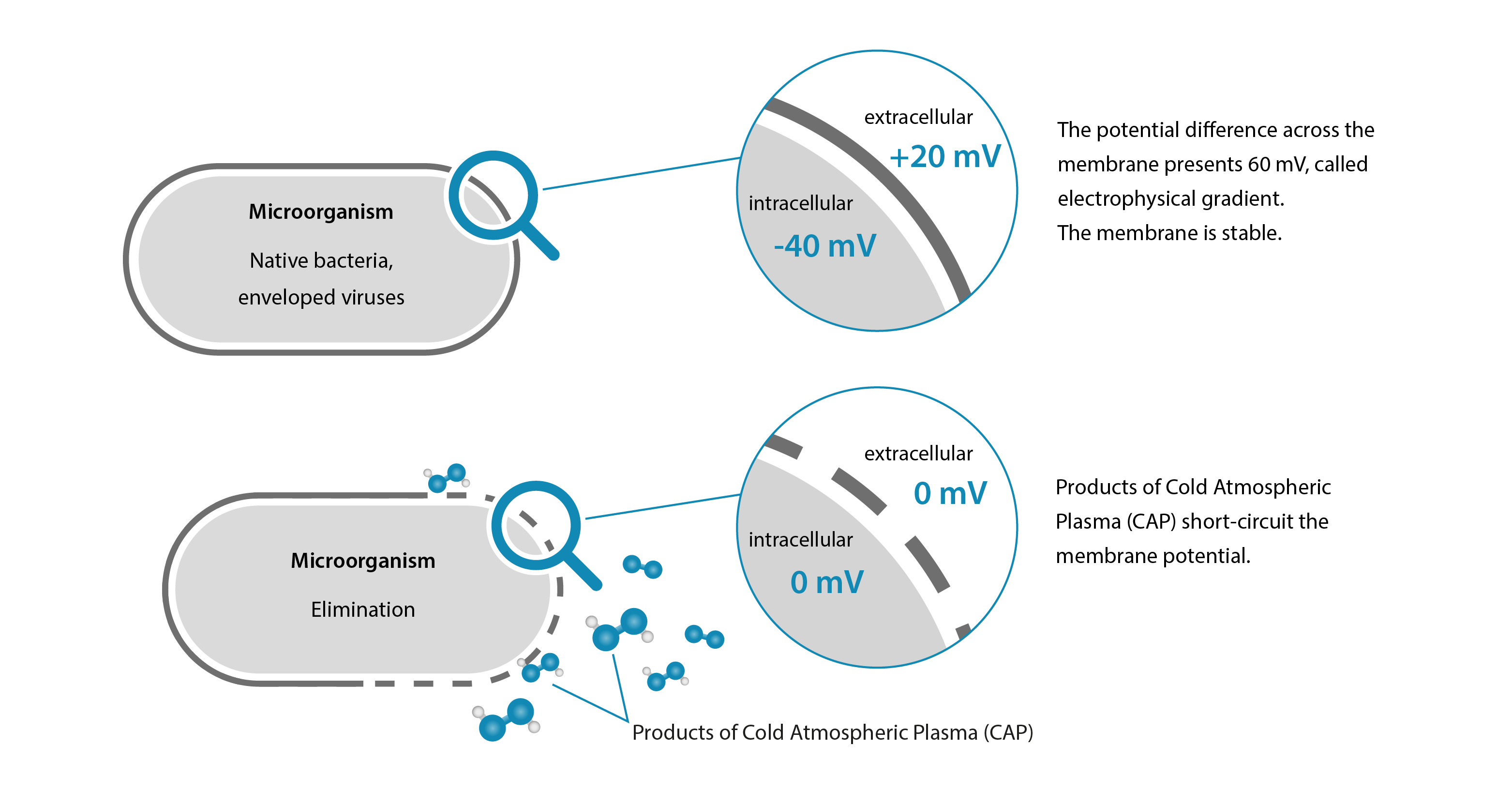
In order to be able to spread cold plasma aerosol using PLASMO® HEAL/PLASMO® VET/PLASMO® HAND, we first need cold plasma ourselves. Our company has developed its own plasma source to produce it. Our unique plasma source requires only electricity and ambient air. This is how cold plasma is produced — an ionized gas. In addition to solid, liquid and gaseous, this gas is referred to as a further, fourth aggregate state. Cold plasma products consist of various charged particles with oxidation potential. These enable the oxidation of microorganisms such as native bacteria, viruses, fungi and multi-resistant germs (MRSA). Cold plasma products are reactive oxygen species which cause a short circuit of the cell membrane of the pathogenic microorganism. Specifically, these particles damage the cell membrane. Redox processes of microorganisms are then disrupted. Pathogenic microorganisms are thus eliminated. Human and animal cells are protected by proteins in their cell network and are therefore not affected — cold plasma is therefore harmless to humans and animals. This germ-killing effect supports the automatic decontamination of the device. In addition to the germ-killing effect of cold plasma, we at WK-Medtec make particular use of the ionization of cold plasma, which becomes relevant in the third step.
Step 2.
Aerosol production.
In PLASMO® HEAL/PLASMO® VET/PLASMO® HAND, a nebulizer module produces a very fine aerosol in the second step. Our aerosol consists of the smallest water particles and ambient air, i.e. water vapor.
Step 3.
The fusion: This is how cold plasma-aerosol/cold atmospheric plasma-aerosol (CAP-A) is produced.
The cold plasma products produced in step 1 hit the aerosol generated in step 2 in a chamber. The oxygen compounds of the cold plasma products electrostatically charge the aerosol. This is made possible by the degree of ionization of the plasma. The patented mixture of cold plasma products and nebulized water (aerosol) promises high effectiveness: The power of the aerosol dispersion is increased.
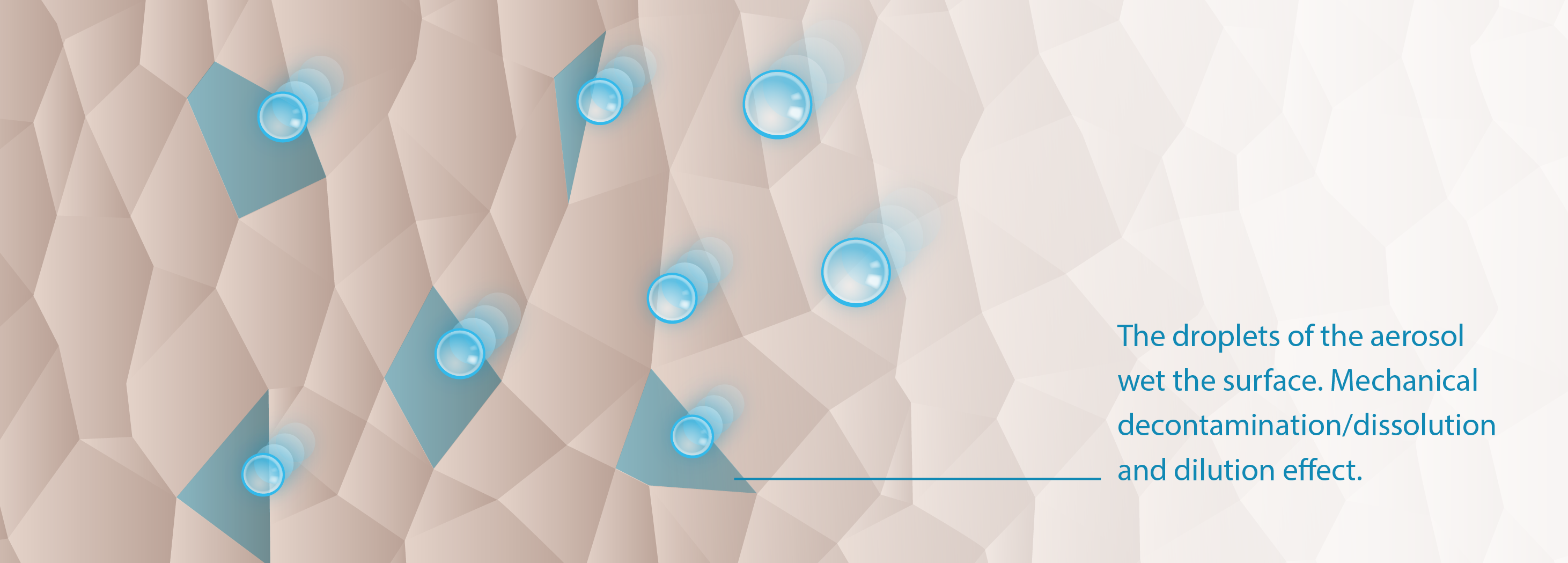
In contact with the skin, the prepared aerosol develops the power of its release and suppression effect in this fourth and final step. This main physical mode of action results in mechanical decontamination of the skin. The graphic below shows how treatment with CAP-A effectively inactivates transient bacteria on the surface. The desired bacteria of the body's own microbiome — the resident flora — are not affected. In addition, side effects such as osmolarity and pH reduction and reactive oxygen species have their effect.
In the conventional direct cold plasma process, cold plasma is produced in a first step, which then comes into contact with the skin when energy is constantly supplied (e.g. “beam”). In contrast, our cold plasma aerosol technology separates energy input and place of action spatially and physically. Energy is only required in the first step to produce cold plasma products at the plasma source. The targeted application of cold plasma aerosol to the skin in the fourth and final step is completely separate from the first step. Our process is different from the direct cold plasma process because it does not involve any energy input into the skin. Energy supply and skin are separate from each other. It is precisely this separation that guarantees pain-free and stress-free care.
This technology offers decisive advantages in terms of effectiveness and application — both in medicine and in industry.
Plasma Disinfection Procedures for Surfaces in Emergency Service Vehicles: A Field Trial at the German Red Cross. Sci Rep 13, 20737.
DOI: https://doi.org/10.1038/s41598-023-47759-5